Texas District Court Applies Loper Bright To Reject FDA’s Unlawful Expansion of Its Regulatory Jurisdiction
By
| April 1, 2025
Yesterday, in American Clinical Laboratory Ass’n v. FDA, a Texas district court properly relied on Loper Bright to reject the FDA’s attempted ultra vires expansion of its jurisdiction under the Food, Drug, and Cosmetic Act (“FDCA”) to regulate, for the first time, laboratory testing services as manufactured “devices.” Ever since City of Arlington, court deference to an agency’s assertion of the scope of its own jurisdiction had been a problem. Loper Bright is the remedy for that ailment.
Background on Lab-Developed Tests
By way of background, lab-developed tests are services administered by trained professionals and do not involve manufacturing. As the district court observed:
Laboratory-developed test services are in-house diagnostic tests developed, validated, and performed by trained professionals within a single clinical laboratory. They are performed on blood, urine, tissue, or other types of specimens at the request of an individual physician, in the context of a specific doctor-patient relationship. Treating doctors rely on such laboratory-developed test services for patient diagnosis, care, and treatment[.]
And “[f]or many years, laboratory-developed test services have been comprehensively regulated by both the States and by the Centers for Medicare and Medicaid Services[.]” But in May 2024 the FDA “issued a final rule . . . announcing its intent to treat all laboratory-developed test services as medical devices and to regulate them under the” FDCA. Plaintiffs subsequently filed a lawsuit challenging this regulation, arguing the FDA didn’t have the statutory authority to regulate testing services as manufactured “devices.”
FDA Attempt to Expand Jurisdiction on Firmer Footing Before Loper Bright
There was once a time when the FDA’s decision to unilaterally expand its regulatory jurisdiction without Congress’s permission might well have stood on firmer footing. It bears reminding that until Loper Bright, federal courts were required under Chevron to defer to federal agencies’ views on what ambiguous statutes mean, so long as those views were deemed “reasonable.” In practice, Chevron deference often meant that the judiciary would rubber-stamp an agency’s power claims. Indeed, the Supreme Court expanded the scope of the Chevron doctrine in 2013 in City of Arlington v. FCC, ruling that federal courts were required to defer to agencies’ assertions of regulatory jurisdiction under certain circumstances.
That all changed in 2024 when the Loper Bright court overruled Chevron and held that courts may no longer “defer to an agency interpretation of the law simply because a statute is ambiguous.” Perhaps seeing the writing on the wall shortly before the Court issued its opinion in Loper Bright, the FDA’s May 2024 regulation (wisely) disclaimed reliance on Chevron deference in response to a comment:
One comment argued that if the Supreme Court overturns or narrows the Chevron doctrine . . . that would “further undermine FDA’s authority to regulate LDTs and further place in question the validity of a final LDT rule.” . . . [T]he Chevron doctrine is not necessary to resolve any question of FDA’s authority over LDTs.
Notably, neither Chevron nor City of Arlington make an appearance in the district court’s decision. Instead, as the district court wrote:
In Loper Bright Enters. v. Raimondo, the Supreme Court made clear that “[c]ourts must exercise their independent judgment in deciding whether an agency has acted within its statutory authority. . . .” The exercise of such independent judgment, the Court explained, is rooted in the “solemn duty” imposed on courts under the Constitution to “say what the law is.” (citations omitted).
The district court continued:
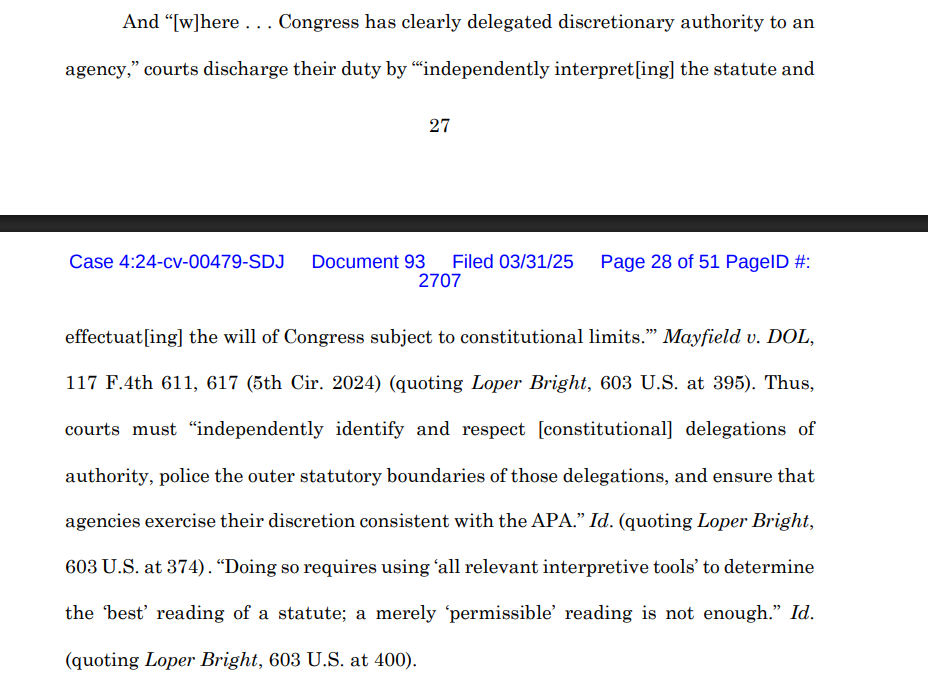
Applying these principles, the district court concluded the FDA’s attempted “expansion” of its “jurisdiction to cover laboratory-developed test services as medical ‘devices’ under the FDCA” is “foreclosed by the text, structure, and history of the FDCA and CLIA.” In other words, the FDA’s regulation is beyond the powers Congress granted to the agency and thus ultra vires and invalid. “Having concluded that the final rule exceeds FDA’s authority and is unlawful,” the district court vacated it.
This decision is a useful illustration of Loper’s impact—even before it was decided. If this case had been decided a decade ago, Chevron and City of Arlington would have been front and center—likely the agency’s lead argument. Today, those cases—and the agency fox-in-the-henhouse syndrome—are no more and Loper Bright rules the roost of statutory interpretation.


